Dr. Enric Llorens Bobadilla | Llorens Bobadilla Lab
Dr. Enric Llorens Bobadilla | Llorens Bobadilla Lab
Bio:
Enric Llorens-Bobadilla is an assistant professor and group leader at the Department of Cell and Molecular Biology at Karolinska Institutet in Stockholm, Sweden. His lab, established in 2022, focuses on understanding glial biology and developing regenerative strategies for the central nervous system, leveraging single-cell and spatial genomics technologies.
Llorens-Bobadilla earned his Ph.D. from the University of Heidelberg and the German Cancer Research Center (DKFZ) in Germany, where he pioneered single-cell transcriptomic approaches to study adult stem cell niches. He then completed postdoctoral training with Jonas Frisén at Karolinska Institutet as a Human Frontier Science Program fellow.
He is the recipient of an ERC Starting Grant, was named a Wallenberg Academy Fellow and received the Swedish Foundation for Strategic Research Future Leaders award.
Abstract:
Injuries to the central nervous system cause permanent disability in mammals because resident cells fail to mount an effective regenerative response. In this talk, I will present our recent studies investigating how glial cells, particularly astrocytes and ependymal cells, respond to spinal cord injury, and what gene-regulatory mechanisms govern their regenerative potential.
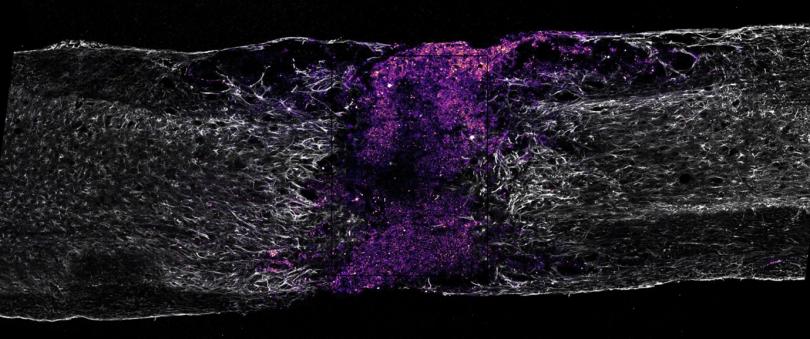
I will first show that ependymal-derived neural stem cells in the injured spinal cord possess a latent lineage potential that, while not manifested under normal conditions, can be unlocked to produce new oligodendrocytes, promote remyelination and restore axon conduction. I will then describe how we mapped the enhancer landscape of the injury response across glial cell types, revealing that injury-responsive enhancers encode cell-type specificity by integrating stress-response and cell-identity transcription factor programs, a logic that enables precision targeting of reactive astrocytes using gene therapy vectors.
Finally, I will present a cross-species comparison at single cell resolution between the regeneration-competent spiny mouse (Acomys) and the laboratory mouse (Mus). While both species activate similar injury-response programs, cells in Acomys rapidly resolve their reactive state whereas those in Mus remain permanently altered. These findings suggest that the reversibility of injury-induced gene-regulatory changes, rather than the initial response itself or a large regulatory rewiring, may be a critical determinant of regenerative success. Together, these studies uncover mechanisms that limit the regenerative potential of glial cells in mammals and identify potential precision interventions to promote spinal cord repair.
Zamboni, M, Martínez Martín, A., Rydholm, G., Häneke, T., Pintado, L., Secilmis. D., Ziegenhain, C., Llorens-Bobadilla, E. (2025) The regulatory code of injury responsive enhancers enables precision cell state targeting in the CNS. Nature Neuroscience. DOI: doi-org.proxy.kib.ki.se/10.1038/s41593-025-02131-w
Llorens-Bobadilla, E.#, Zamboni, M., Marklund, M., Bhalla, N., Chen, X., et al. (2023). Solid-phase capture and profiling of open chromatin by spatial ATAC. Nature Biotechnology, 41(8), 1085-1088.
Llorens-Bobadilla, E., Chell, J.M., Le Merre, P., Wu, Y., Zamboni, M., et al. (2020). A latent lineage potential in resident neural stem cells enables spinal cord repair. Science, 370(6512), eabb8795.
View Dr. Llorens Bobadilla CV here!