Fauna BIO
Fauna BIO has an interesting business model that seeks to exploit the basic science of special animal phenotypes for insight into the development of new therapeutics (think GLP-1 agonists).
Click here to learn more about Fauna Bio.
Fauna BIO has an interesting business model that seeks to exploit the basic science of special animal phenotypes for insight into the development of new therapeutics (think GLP-1 agonists).
Click here to learn more about Fauna Bio.

Join Alumni, friends, and members of the Department of Biology to celebrate our Annual Biology Alumni Weekend featuring the following opportunities below.
16th Annual Thomas Hunt Morgan lecturer, Dr. Alejandro Sánchez Alvarado of the Stower's Institute, will provide a scientific lecture, "Understanding the Sources of Regenerative Capacity in Animals," at 2 p.m. in the Thomas Hunt Morgan building, room 116.
Coffee & Networking - Meet current undergraduate and graduate students and connect with fellow alumni to share your career experience. 10-11 a.m., W.T. Young Library, Multipurpose Room (B-108C), 401 Hilltop Ave., Lexington, KY 40508
Meet Current Biology Faculty - Come learn about the exciting research and innovative teaching going on in our department from 11:30 a.m. -12:30 p.m. in the Don and Cathy Jacobs Science Building, Room 361M, 680 Rose St., Lexington, KY 40508
Thomas Hunt Morgan Reception - Drinks and food with fellow alumni and members of the department at the historic Thomas Hunt Morgan House in downtown Lexington, 210 North Broadway, 40507 from 6 p.m. to 9 p.m. Check out the fun we had in previous years here.
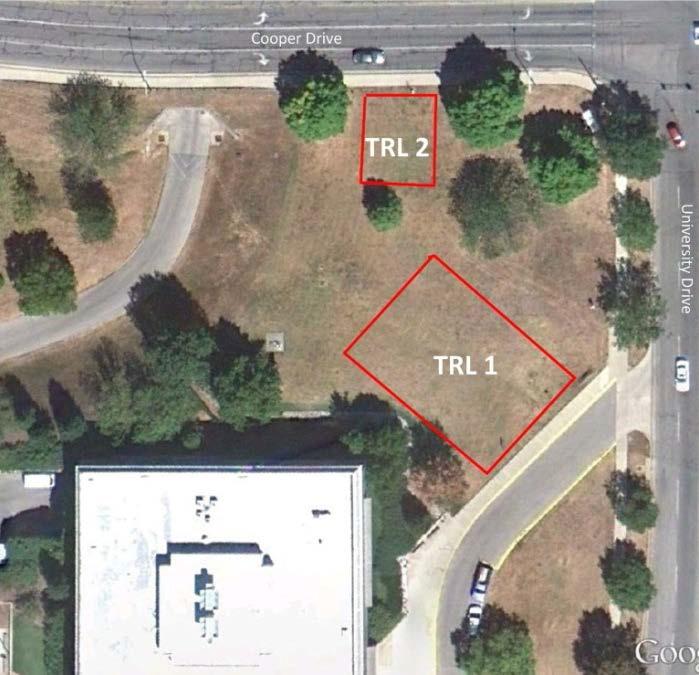
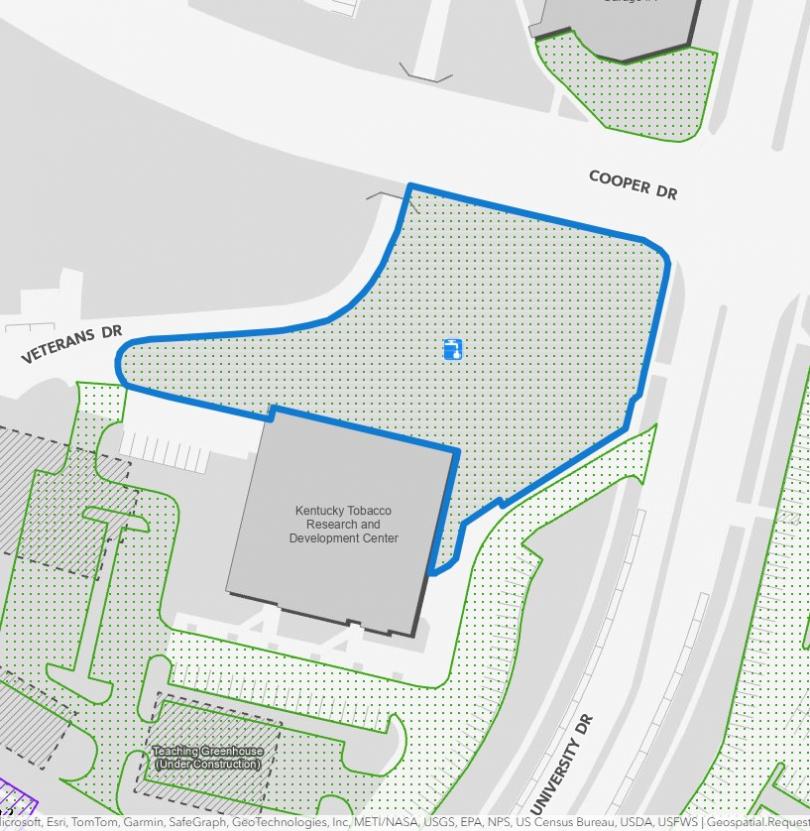
Alumni Tailgate - UK vs. Tennessee. 5 p.m., game starts at 7:45pm Location: Tobacco Research Lawns, 1401 University Drive, Lexington, KY 40503
Pictures of the tailgate location below.
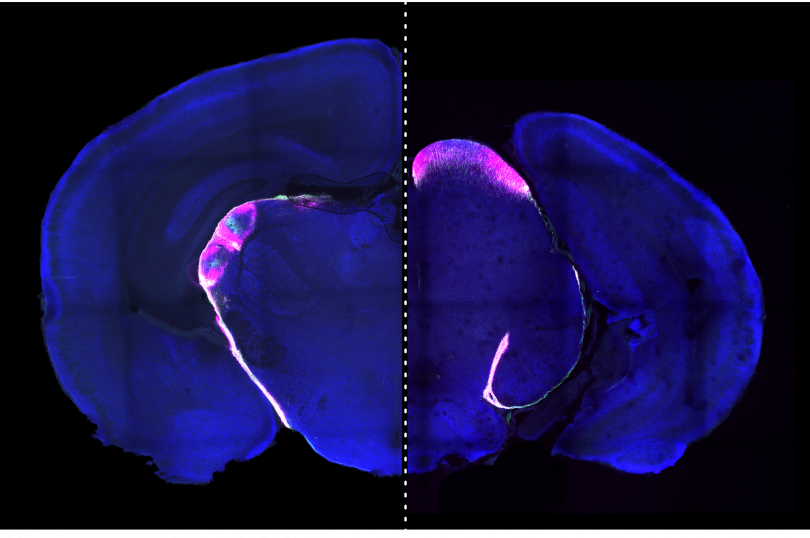
*The Thomas Hunt Morgan lecture was created in 2009 to honor the research career of Thomas Hunt Morgan, a Lexington native and graduate of the University of Kentucky, and to honor the outstanding scientists whose work reflects the highest achievement in genetics, molecular biology, and developmental biology. This lecture gives scientists the opportunity to highlight their work to basic researchers, physician scientists, research faculty, postdoctoral fellows, and graduate and undergraduate students.
 Dr. Nick Keiser | Keiser Lab
Dr. Nick Keiser | Keiser LabBio:
Nick Keiser is a behavioral disease ecologist interested in how behavioral trait variation can influence infectious disease dynamics. His mentees study questions at the nexus of animal behavior, parasitology and disease ecology in a variety of such (mostly invertebrate) study systems as flies, spiders, ticks, snails and their associated parasites.
Abstract:
The fields of animal behavior and infectious disease are both typified by heterogeneity. Differences among individuals, between social groups and between populations in their behavioral trait compositions can all alter the dynamics of infectious diseases. In this seminar, I will address how animal behavior can alter host-pathogen interactions across different scales from individuals to populations in several study systems. These study systems will weave three tales on behavioral trait diversity, behavioral parasitology, and parasite manipulation of host behavior.
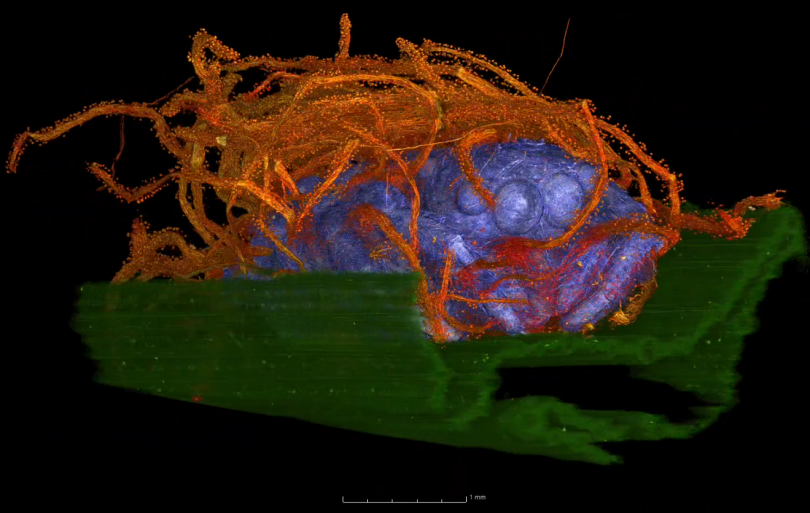

Bio:
Afshin Beheshti, Ph.D. is a professor of surgery and of computational and systems biology at the University of Pittsburgh. He serves as director of the newly launched Space Center for Space Biomedicine and as associate director of the McGowan Institute for Regenerative Medicine at Pitt. In addition, Beheshti holds a visiting scientist appointment at the Broad Institute of MIT and Harvard.
Abstract:
Human spaceflight presents significant health challenges driven by microgravity, space radiation, isolation and other environmental stressors. Recent multi-omics research has revealed that mitochondrial dysfunction is a central biological consequence of space travel, contributing to systemic impacts such as accelerated aging, cardiovascular disease, and impaired metabolic function. Data from astronaut missions and ground-based space analogs demonstrate persistent mitochondrial suppression even after returning to Earth. This talk highlights how space serves as an accelerated model for studying human diseases and aging, offering insights applicable both to space exploration and terrestrial medicine. Using advanced 3D organoid models and multi-omics analysis, we have identified promising countermeasures, including the natural flavonoid Kaempferol, which restores mitochondrial bioenergetics and reverses radiation-induced gene expression changes in multiple tissues. These findings underscore the critical role of mitochondria as both biomarkers and therapeutic targets for sustaining human health in deep space missions, while also advancing precision medicine strategies on Earth.
 Dr. Antonis Rokas | Rokas Lab
Dr. Antonis Rokas | Rokas LabBio:
Antonis Rokas is a professor at the Departments of Biological Sciences and of Biomedical Informatics at Vanderbilt University and a holder of the Cornelius Vanderbilt Chair in Biological Sciences. He also serves as the founding director of the Vanderbilt Evolutionary Studies Initiative (http://www.vanderbilt.edu/evolution), an interdisciplinary center that unites scholars from diverse disciplines with broad interests and expertise in evolution-related fields. Rokas received his B.S. in Biology from the University of Crete, Greece (1998) and his Ph.D. from Edinburgh University, Scotland (2001). Before joining Vanderbilt in the summer of 2007, he was a postdoctoral fellow at the University of Wisconsin-Madison (2002–2005) and a research scientist at the Broad Institute (2005–2007). Research in the Rokas lab focuses on the study of the DNA record to gain insight into the patterns and processes of evolution. Through a combination of computational and experimental approaches, his current research aims to understand the molecular foundations of the fungal lifestyle, the reconstruction of the tree of life and the evolution of human pregnancy-associated traits.
Abstract:
Eutherian mammals have characteristic lengths of gestation that are key for reproductive success, but relatively little is known about the processes that determine the timing of parturition. This issue remains one of biology's great unsolved mysteries and has significant clinical relevance because preterm birth is the leading cause of infant and under 5-year-old child mortality worldwide. In my talk, I will describe my team’s and collaborative efforts to understand the genetic architecture of gestation length in (European) humans, the evolutionary forces acting on human loci involved (and an approach for extending this for any complex trait), and the evolution of gestation length in relation to other life history traits across mammals.
 Dr. Deb Bell-Pedersen | Pedersen Lab
Dr. Deb Bell-Pedersen | Pedersen LabAbstract:
The circadian clock is a fundamental regulator of human health and drug metabolism, coordinating daily rhythms in protein production that affect cellular function and metabolism. Many proteins that cycle robustly are produced from nonrhythmic mRNAs, pointing to translational control as a key mechanism of rhythmic protein levels. Using the model eukaryote Neurospora crassa, we discovered that the clock exerts this regulation through rhythmic control of a conserved translation initiation factor (eIF2α) and by remodeling ribosome composition. Even more unexpectedly, we discovered that the circadian system governs the fidelity of protein synthesis by modulating ribosome makeup and tRNA synthetase activity. Both translational fidelity and circadian amplitude decline with age. We identified compounds that restore clock amplitude in old N. crassa cells, leading to improved translation accuracy and extended lifespan. These findings reveal how the circadian clock programs daily changes in the proteome beyond genomic instructions and highlight a novel link among circadian regulation, proteome integrity and aging.
Bio:
Sánchez Alvarado received a B.S. in molecular biology and chemistry from Vanderbilt University in Nashville, Tennessee, and a Ph.D. in pharmacology and cell biophysics from the University of Cincinnati College of Medicine. He performed postdoctoral and independent research at the Carnegie Institution of Washington, Department of Embryology in Baltimore. In 2002, he joined the faculty of the University of Utah School of Medicine in Salt Lake City, where he held the H.A. & Edna Benning Presidential Endowed Chair. In 2005, he was named a Howard Hughes Medical Institute investigator. He joined the Stowers Institute for Medical Research in Kansas City in 2011 and became the president and chief scientific officer of the Stowers Institute in 2022. He also holds the Priscilla Wood Neaves Chair in the Biomedical Sciences.
Sánchez Alvarado is an elected member of the National Academy of Science, the American Academy of Arts and Sciences and the Latin American Academy of Sciences; a Kavli Fellow of the National Academy of Sciences USA; a fellow of the Marine Biological Laboratory in Woods Hole, MA; a fellow of the American Association for the Advancement of Science; and a recipient of a National Institutes of Health MERIT award, the EE Just Medal for Scientific Achievement and the Vilcek Prize in Biomedical Sciences. He has served on numerous scientific advisory committees and boards including the National Advisory Council of the National Institute of General Medical Sciences and the National Institutes of Health. He serves on the Board of Directors of American Century Investments.
Sánchez Alvarado’s work has the potential to lead to a better understanding of how the adult forms of higher organisms, including humans, carry out their biological functions. His research also has led to insights on the molecular and genetic drivers of both regenerative and degenerative cellular processes that contribute to disease.
Abstract:
It is paradoxical that for many organisms (including humans), the apparent anatomical stability of their adult bodies is maintained by constant change. Despite the importance of tissue homeostasis and regeneration to human biology and health, relatively little is known about how these processes are regulated. As such, numerous questions remain, including:
Answering any of these questions would set a baseline from which to try to enhance regenerative properties in multicellular organisms such as humans, particularly after injury.
One way to solve a complex problem is to reduce it to a simpler, easier way to answer problem. Therefore, reducing the complexities of regeneration and tissue homeostasis to the study of comparatively simpler systems would allow for a systematic dissection and mechanistic understanding of these processes. Here, I will discuss how the use of single-cell and spatial transcriptomics is helping define the cellular and molecular environments that support pluripotency in the highly regenerative freshwater planarian Schmidtea mediterranea and regeneration of missing organs in the hemichordate Ptychodera flava. Our studies are beginning to shed light on the way adult animals regulate tissue homeostasis and the replacement of body parts lost to injury.
 Dr. Yvon Woappi | Woappi Lab
Dr. Yvon Woappi | Woappi LabBio:
Dr. Yvon Woappi is the Herbert and Florence Irving Assistant Professor of Physiology and Cellular Biophysics, Dermatology, and Biomedical Engineering at Columbia University. His research leverages gene editing and multiomic technologies to uncover how autonomous multicellular orchestration facilitates deep wound repair – a process critical to many conditions including diabetic ulcers and carcinomas. Dr. Woappi earned his Ph.D. as a Grace Jordan McFadden Fellow at the University of South Carolina and completed his postdoctoral training in the Harvard Dermatology Research Training Program at Harvard Medical School. Dr. Woappi’s pioneering early-career research is laying the foundation for synthetic wound regeneration, a systems bioengineering approach that leverages cellular heterogeneity to enhance tissue regeneration.
Abstract:
As the organ most frequently exposed to predatory pressures, the integument has acquired broad functions, including camouflage, thermoregulation, sensory perception, and tissue repair. These roles are executed through a complex interplay of tissue substructures, including several mini-organ appendages (hair follicles, sebaceous glands, arrector pili muscle, and assorted pilosebaceous units) and five central adnexal structures (blood vessels, sensory neurons, collagenous tissues, immune components, and deep fascia), all embedded within three superimposed tissue strata (the epidermis, dermis, and hypodermis). Given this intricate architecture, the healing of deep skin wounds requires a coordinated organ-level response involving varied cell populations originating from virtually all three embryonic germ layers—ectoderm, mesoderm, and endoderm. However, a comprehensive understanding of the cellular and molecular logic orchestrating this crosstissue response in mammals remains incomplete. Here, we present the Organ-Scale Wound Healing Atlases (OWHA), a comprehensive multiomic single-cell and spatial transcriptomic dataset that captures the dynamic microanatomical tissue niches of the mammalian integument during the entire wound healing sequence, including early and late healing phases. By incorporating multi-omics data across all major phases of healing, OWHA uncovered novel emergent healing cell states and their coordinated cell fate decisions uniquely (multilineage crosstalk) executed after injury, and delineated critical tissue trajectories required for eKective healing of deep wounds. Importantly, comparative analysis between human and mouse revealed conserved network between the epithelial and neuro-endothelial vasculature....(Add missing groups in human only found in our multi modal approach) By providing deeper mechanistic insights of mammalian tissue adaptations for injury response, OWHA serves as a valuable resource for understanding the cellular and molecular mechanisms underlying wound healing in the mammalian integument.
 Dr. Georgia Hodes
Dr. Georgia Hodes Bio:
Dr. Hodes received a B.A. in Drama/Dance from Bard College and after college worked as an actor and designer in New York City. During this time, she decided a life in the arts was unsustainable and did post-baccalaureate training at Hunter College in experimental psychology. She obtained her Ph.D. from Rutgers University in the Behavioral and Systems Neuroscience division of the Psychology program where she trained in the laboratory of Dr. Tracey Shors. She went on to have 2 postdoctoral training positions, the first in Pharmacology with Dr. Irwin Lucki at the University of Pennsylvania and the second in Neuroscience with the Dr. Scott Russo at the Icahn School of Medicine at Mt. Sinai. She has received 2 NARSAD young investigator awards and is an author on over 70 publications. In 2016 she joined the faculty of the newly formed School of Neuroscience at Virginia Tech. Her research program examines sex differences in the peripheral and central immune system and how immune mechanisms interact with brain plasticity to drive behavioral differences in emotional processing of stress and mental health disorders.
Abstract:
The brain does not exist in a vacuum. The brain controls the body; however, the body also impacts the brain. This occurs through a variety of immune mechanisms, including cytokines and microbes produced in the periphery. When we get sick, our cells produce or suppress signals depending on the type of invasion. They tailor the environment to kill off an invading pathogen or our own injured cells. Cells then produce other signals to shut down this response and promote recovery and healing. Like physical illness, mental illness, or even stress exposure, alters immune signaling in both the body and the brain. The fundamental question driving my research is how immune pathways contribute to the pathology associated with the development of mental illness, and why are some individuals more vulnerable than others to this type of immune dysfunction?
Watch the seminar here!
 Dr. Shane D'Souza
Dr. Shane D'SouzaBio:
Dr. Shane Peter D’Souza is a neuroscientist and vision researcher whose work spans developmental neurobiology, sensory physiology, and circadian biology. He earned his BS in Biology at the University of Kentucky and PhD in Molecular and Developmental Biology from the University of Cincinnati/Cincinnati Children’s Hospital Medical Center, where he investigated how early light exposure shapes neural circuit development in the retina and brain. Now a Postdoctoral Research Fellow in Pediatric Ophthalmology at Cincinnati Children’s, Dr. D’Souza’s research integrates molecular, anatomical, physiological, and computational approaches to understand how intrinsically photosensitive retinal ganglion cells (ipRGCs) and other non-visual photoreceptors influence sensory-driven circuit refinement, photoreceptor abundance, and cross-modal communication in early life. His publication record includes discoveries on melanopsin-dependent regulation of rod photoreceptors, neuropsin and encephalopsin function in visual and non-visual systems, and the role of light-sensitive hypothalamic neurons in thermoregulation and metabolism. By combining high-resolution imaging, transcriptomics, biophysical modeling, and machine-learning, his work aims to uncover fundamental principles of sensory-mediated neural development and sensory adaptation across species. In his spare time, he enjoys studying the evolution of storm systems and serves as a storm spotter for NWS Wilmington, OH. In his spare-spare time, he writes and produces music from his living room.
Abstract:
Most mammals are born with immature, poorly developed sensory systems. As these systems come online, they use immature sensory experience to shape synapses, cell types, and their connectivity across the brain. In the visual system, this experience is thought to be passive, supporting and setting up later modes of image-forming vision after eye-opening. However, little is known about the form and function of visual experience during the earliest period in a neonate’s life. Driven by this, we set out to generate a comprehensive map of visual system activity and neonatal behaviors in mice. Using a host of machine learning-based approaches we developed an atlas perinatal visual system activity from the retina to several regions of the brain. Using a combination of chromatic stimuli and genetic loss-of-function mice, we identified the M1 intrinsically photosensitive retinal ganglion cell (ipRGC) in the retina as the driver of early visual system activity, activating distinct brain regions during development. Using this atlas as a guide to assess behaviors, we find that this visual input drives the production of ultrasonic vocalization in neonates and “blinding” mice leads to an augmented vocal code. Together, these data suggest that early visual system activity has an active role in supporting the development of neonatal behaviors and warrants a deeper exploration of early sensory activity across the developing brain.
Watch the seminar here!